Arsenic Trioxide Induces Apoptosis of Human Monocytes during Macrophagic Differentiation through Nuclear Factor-κB-Related Survival Pathway Down-Regulation
Por um escritor misterioso
Last updated 14 fevereiro 2025

Arsenic trioxide (As2O3) is known to be toxic toward leukemia cells. In this study, we determined its effects on survival of human monocytic cells during macrophagic differentiation, an important biological process involved in the immune response. As2O3 used at clinically relevant pharmacological concentrations induced marked apoptosis of human blood monocytes during differentiation with either granulocyte-macrophage colony-stimulating factor or macrophage colony-stimulating factor. Apoptosis of monocytes was associated with increased caspase activities and decreased DNA binding of p65 nuclear factor-κB (NF-κB); like As2O3, the selective NF-κB inhibitor ( E )-3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile (Bay 11-7082) strongly reduced survival of differentiating monocytes. The role of NF-κB in arsenic toxicity was also studied in promonocytic U937 cells during phorbol 12-myristate 13-acetate-induced macrophagic differentiation. In these cells, As2O3 first reduced DNA binding of p65 NF-κB and subsequently induced apoptosis. In addition, overexpression of the p65 NF-κB subunit, following stable infection with a p65 retroviral expressing vector, increased survival of As2O3-treated U937 cells. As2O3 specifically decreased protein levels of X-linked inhibitor of apoptosis protein and FLICE-inhibitory protein, two NF-κB-regulated genes in both U937 cells and blood monocytes during their differentiations. Finally, As2O3 was found to inhibit macrophagic differentiation of monocytic cells when used at cytotoxic concentrations; however, overexpression of the p65 NF-κB subunit in U937 cells reduced its effects toward differentiation. In contrast to monocytes, well differentiated macrophages were resistant to low concentrations of As2O3. Altogether, our study demonstrates that clinically relevant concentrations of As2O3 induced marked apoptosis of monocytic cells during in vitro macrophagic differentiation likely through inhibition of NF-κB-related survival pathways.
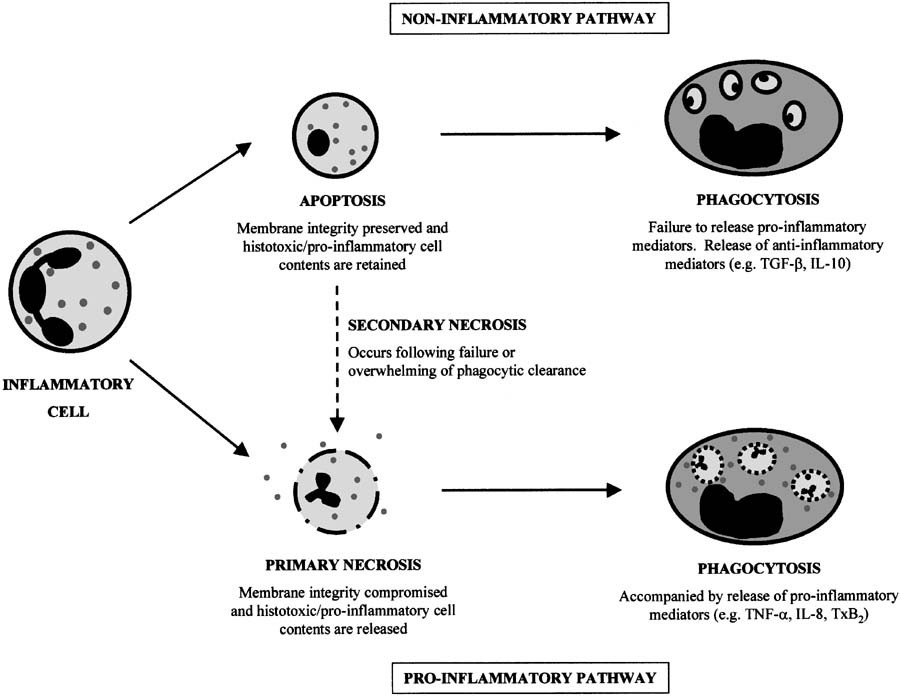
Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis
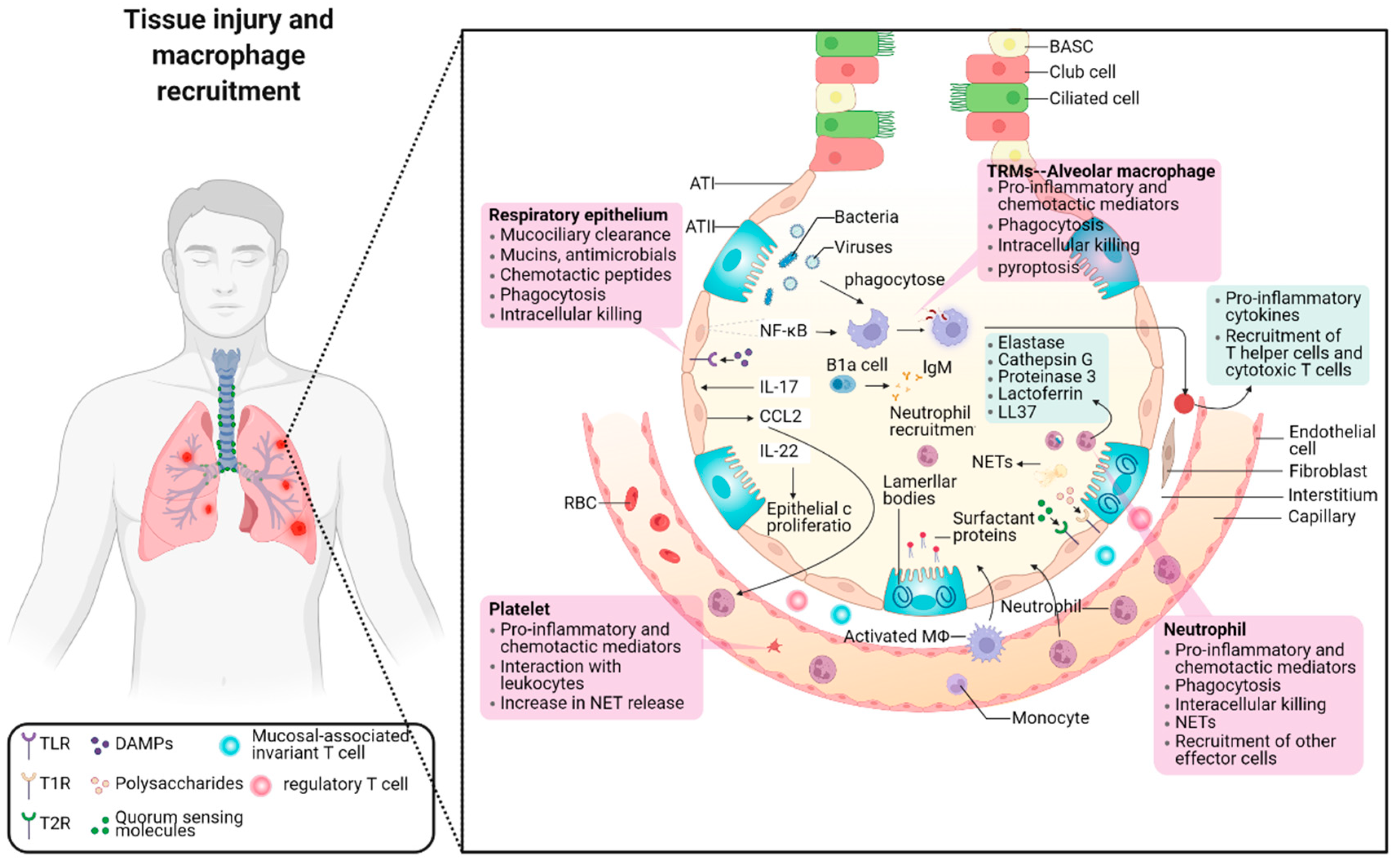
IJMS, Free Full-Text

Arsenic trioxide-induced p38 MAPK and Akt mediated MCL1 downregulation causes apoptosis of BCR-ABL1-positive leukemia cells - ScienceDirect
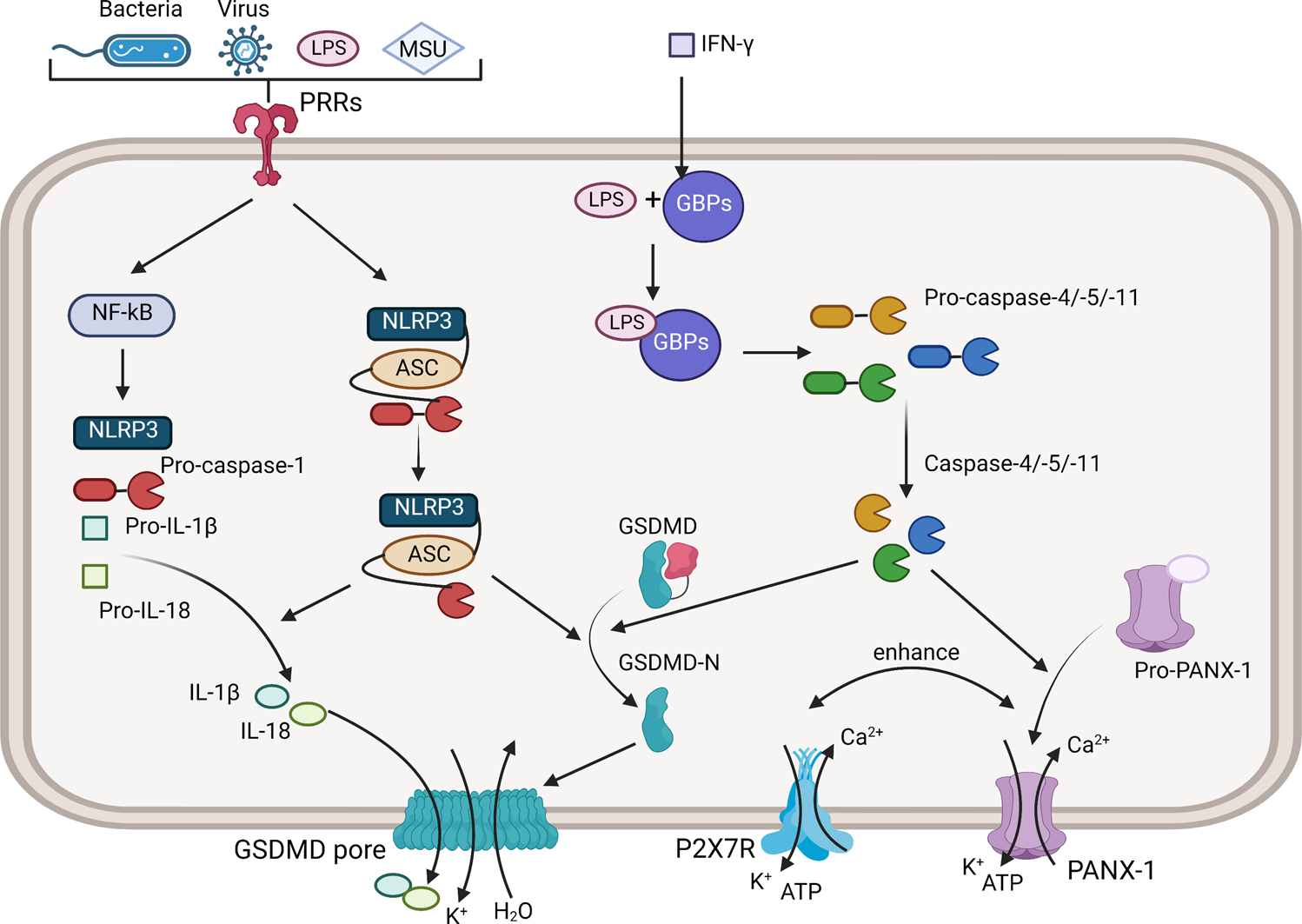
The regulatory role and therapeutic application of pyroptosis in musculoskeletal diseases
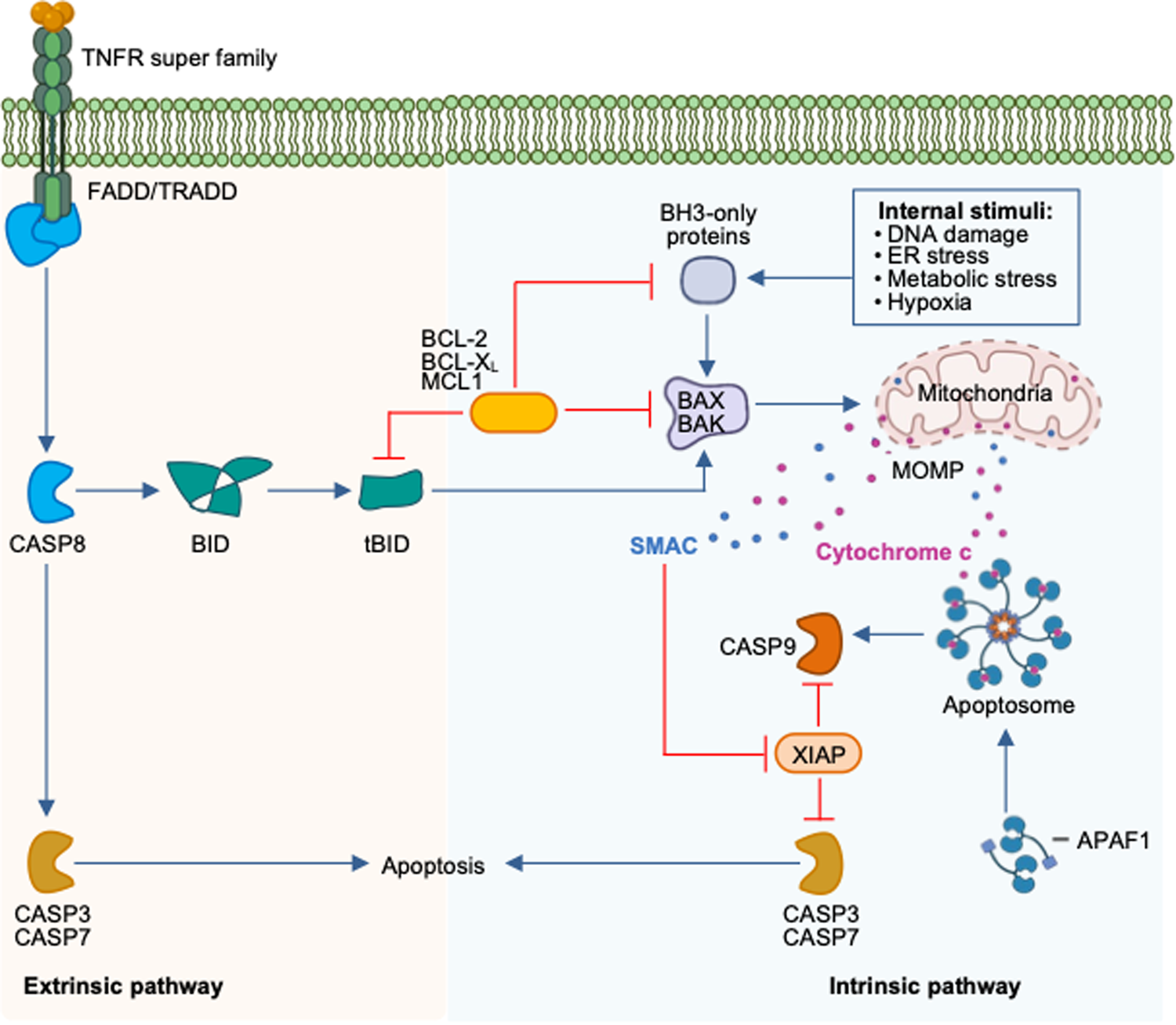
Regulated cell death pathways and their roles in homeostasis, infection, inflammation, and tumorigenesis
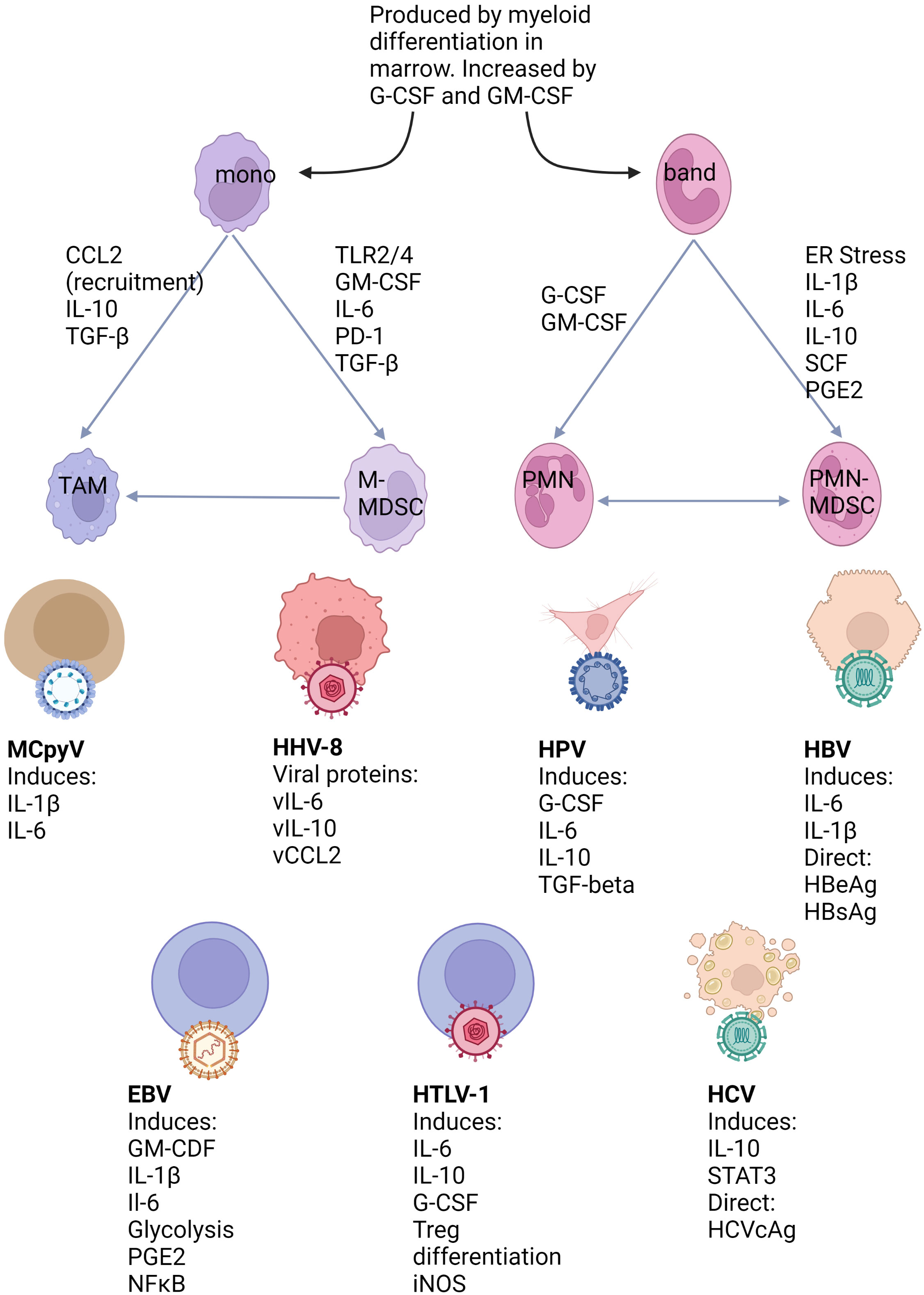
Frontiers Deciphering the roles of myeloid derived suppressor cells in viral oncogenesis
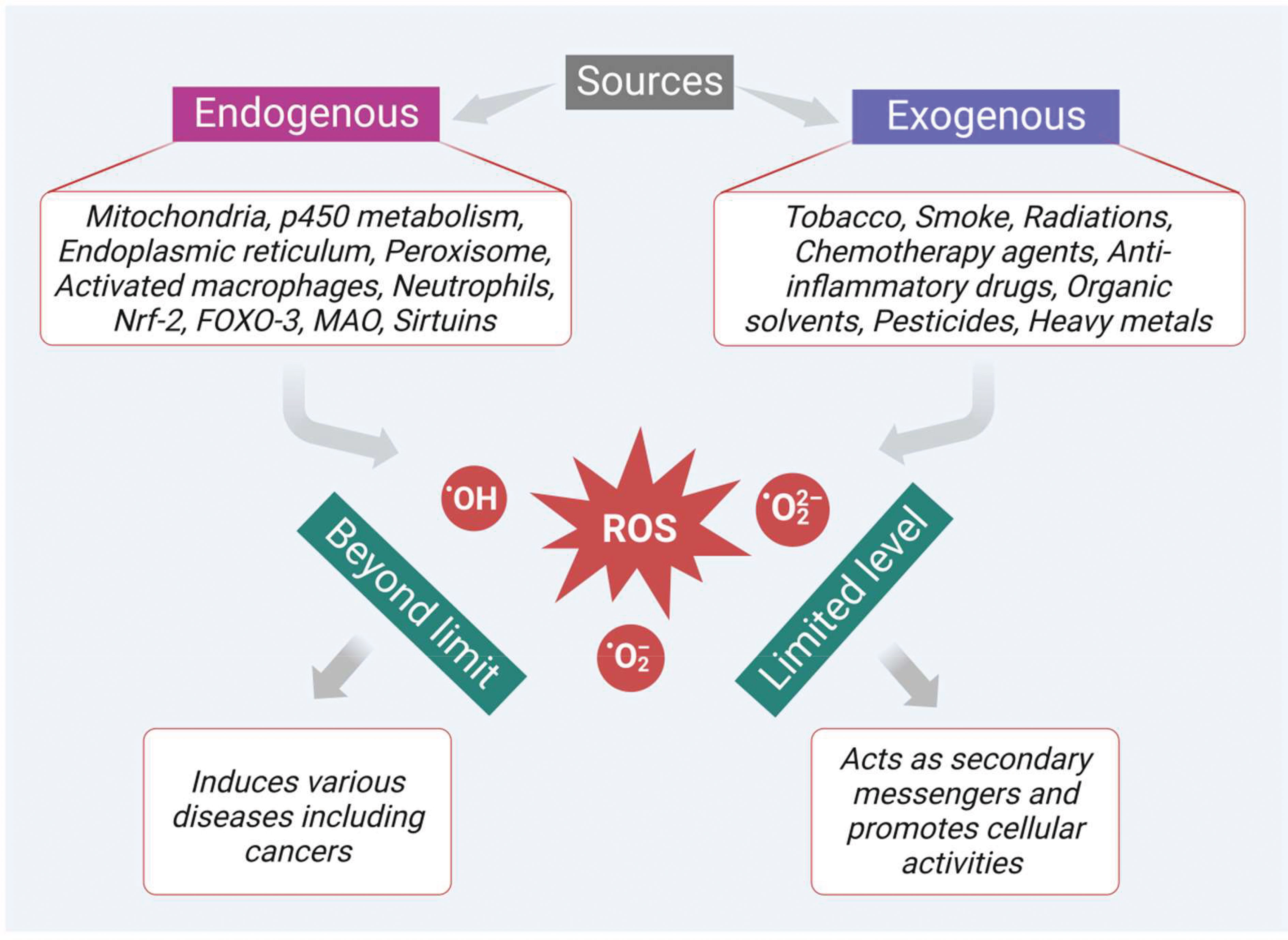
Frontiers Reactive oxygen species mediated apoptotic death of colon cancer cells: therapeutic potential of plant derived alkaloids

Phytochemicals targeting NF-κB signaling: Potential anti-cancer interventions - ScienceDirect
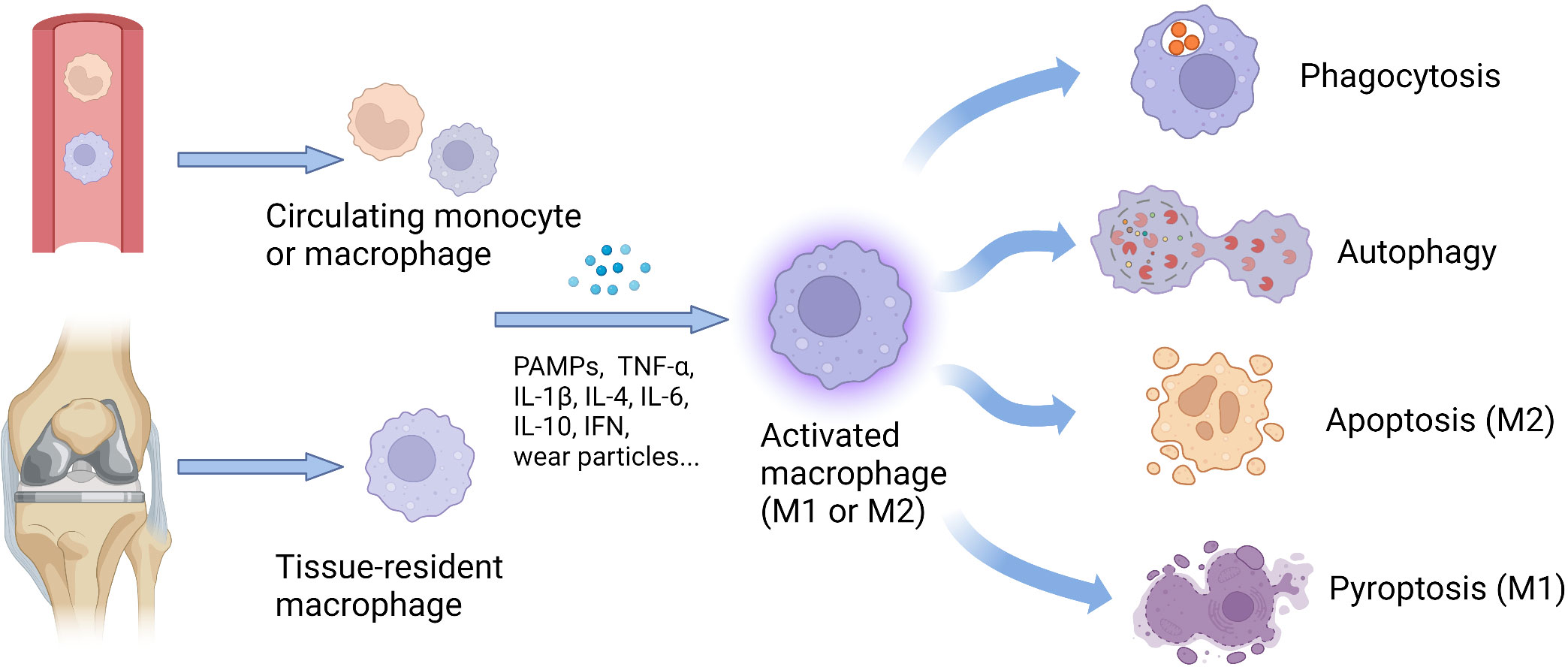
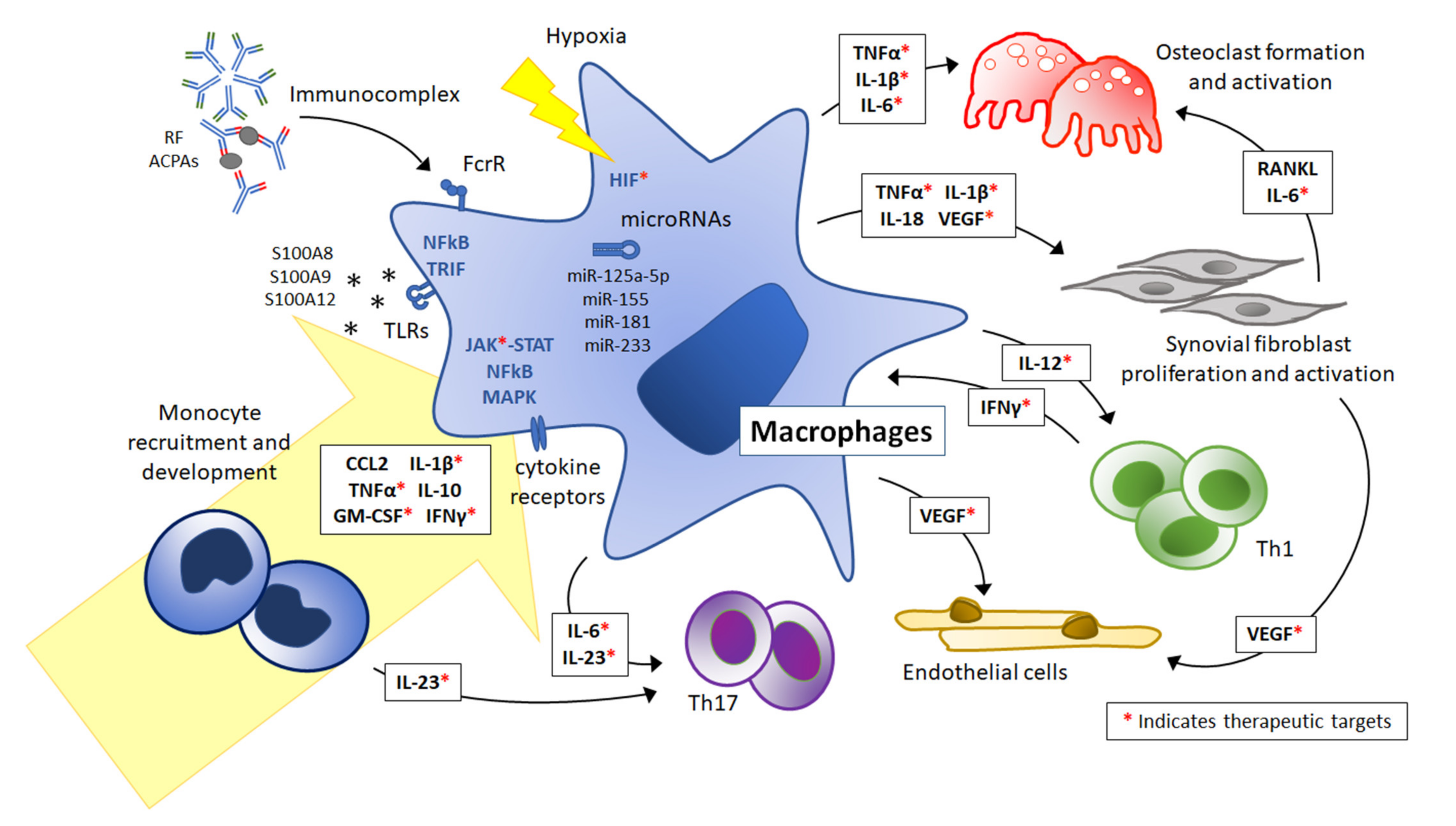
Frontiers Macrophages in aseptic loosening: Characteristics, functions, and mechanisms

Arsenic trioxide induces regulatory functions of plasmacytoid dendritic cells through interferon-α inhibition - ScienceDirect
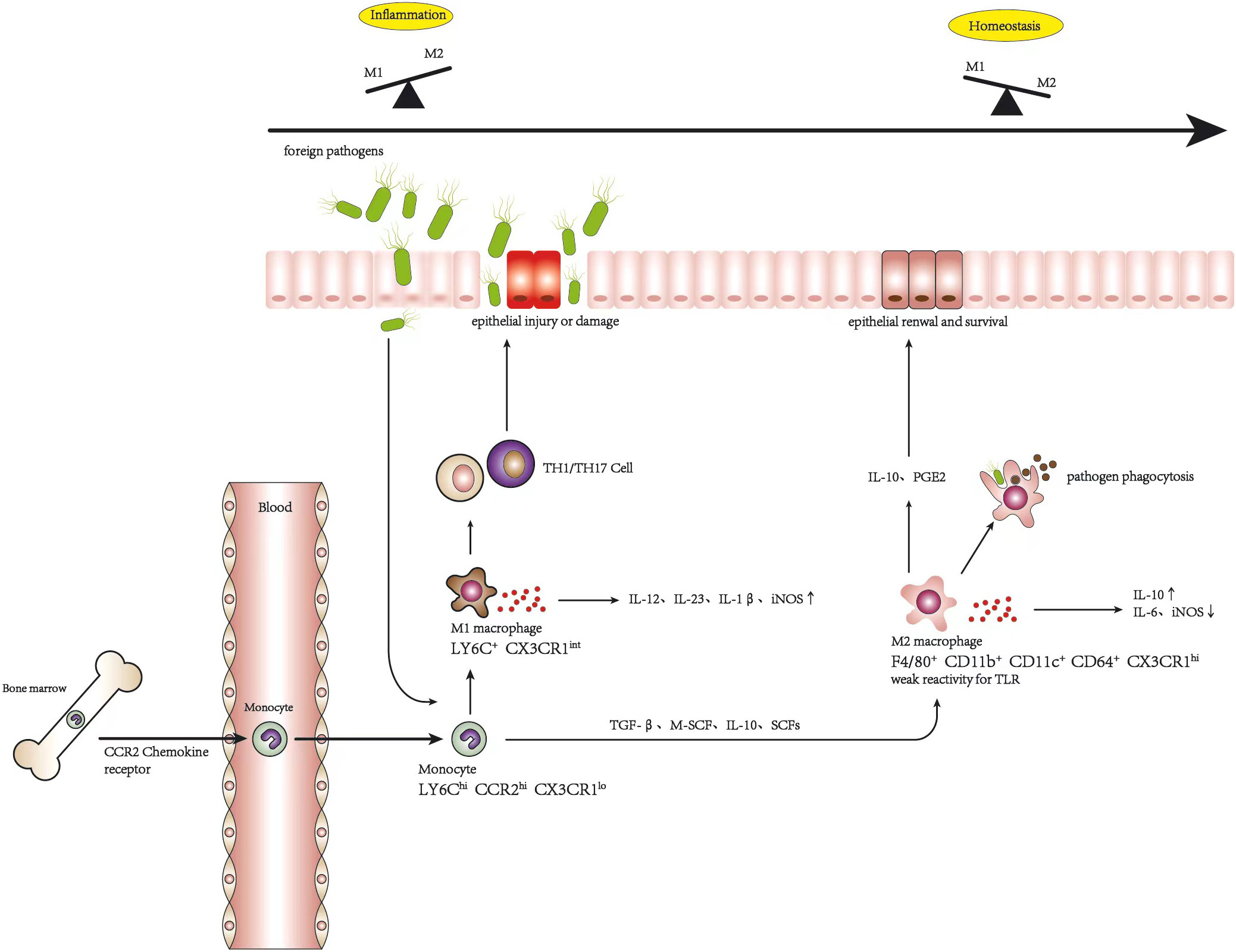
Frontiers A potential therapeutic approach for ulcerative colitis: targeted regulation of macrophage polarization through phytochemicals
IJMS, Free Full-Text

Macrophage Reprogramming via Targeted ROS Scavenging and COX-2 Downregulation for Alleviating Inflammation

Arsenic Trioxide Induces Apoptosis of Human Monocytes during Macrophagic Differentiation through Nuclear Factor-κB-Related Survival Pathway Down- Regulation

Arsenic trioxide induces regulatory functions of plasmacytoid dendritic cells through interferon-α inhibition - ScienceDirect
Recomendado para você
-
 Most Disturbed Person on Planet Earth III (2019) - IMDb14 fevereiro 2025
Most Disturbed Person on Planet Earth III (2019) - IMDb14 fevereiro 2025 -
 MDPOPE: albums, songs, playlists14 fevereiro 2025
MDPOPE: albums, songs, playlists14 fevereiro 2025 -
 Americas Sickest Home Videos (DVD, 2011) for sale online14 fevereiro 2025
Americas Sickest Home Videos (DVD, 2011) for sale online14 fevereiro 2025 -
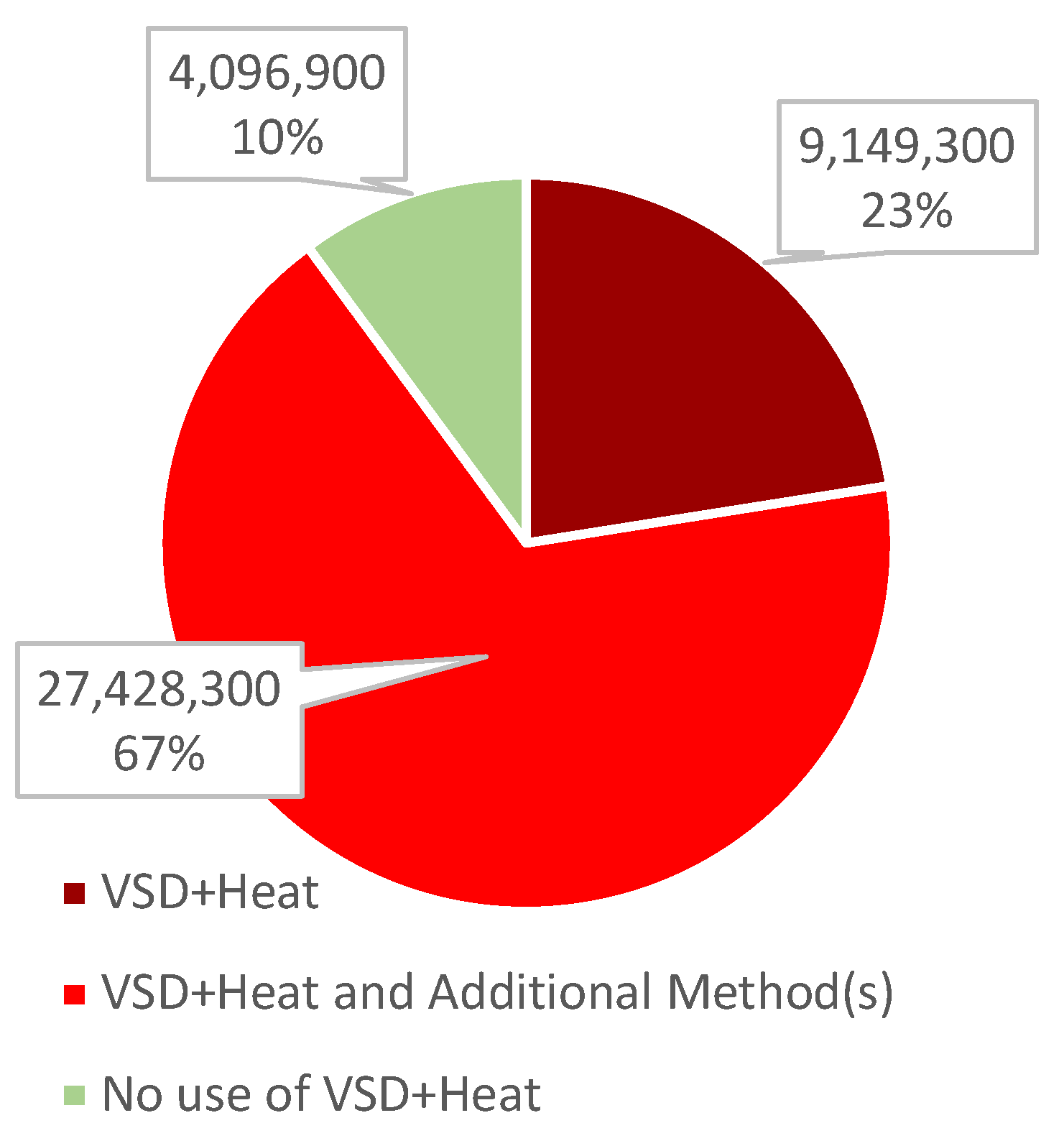 Animals, Free Full-Text14 fevereiro 2025
Animals, Free Full-Text14 fevereiro 2025 -
 Stream Faces Of The Voices (Feat. @MDPOPE) by outcas214 fevereiro 2025
Stream Faces Of The Voices (Feat. @MDPOPE) by outcas214 fevereiro 2025 -
 Xeritin - MDPOPE MP3 Download & Lyrics14 fevereiro 2025
Xeritin - MDPOPE MP3 Download & Lyrics14 fevereiro 2025 -
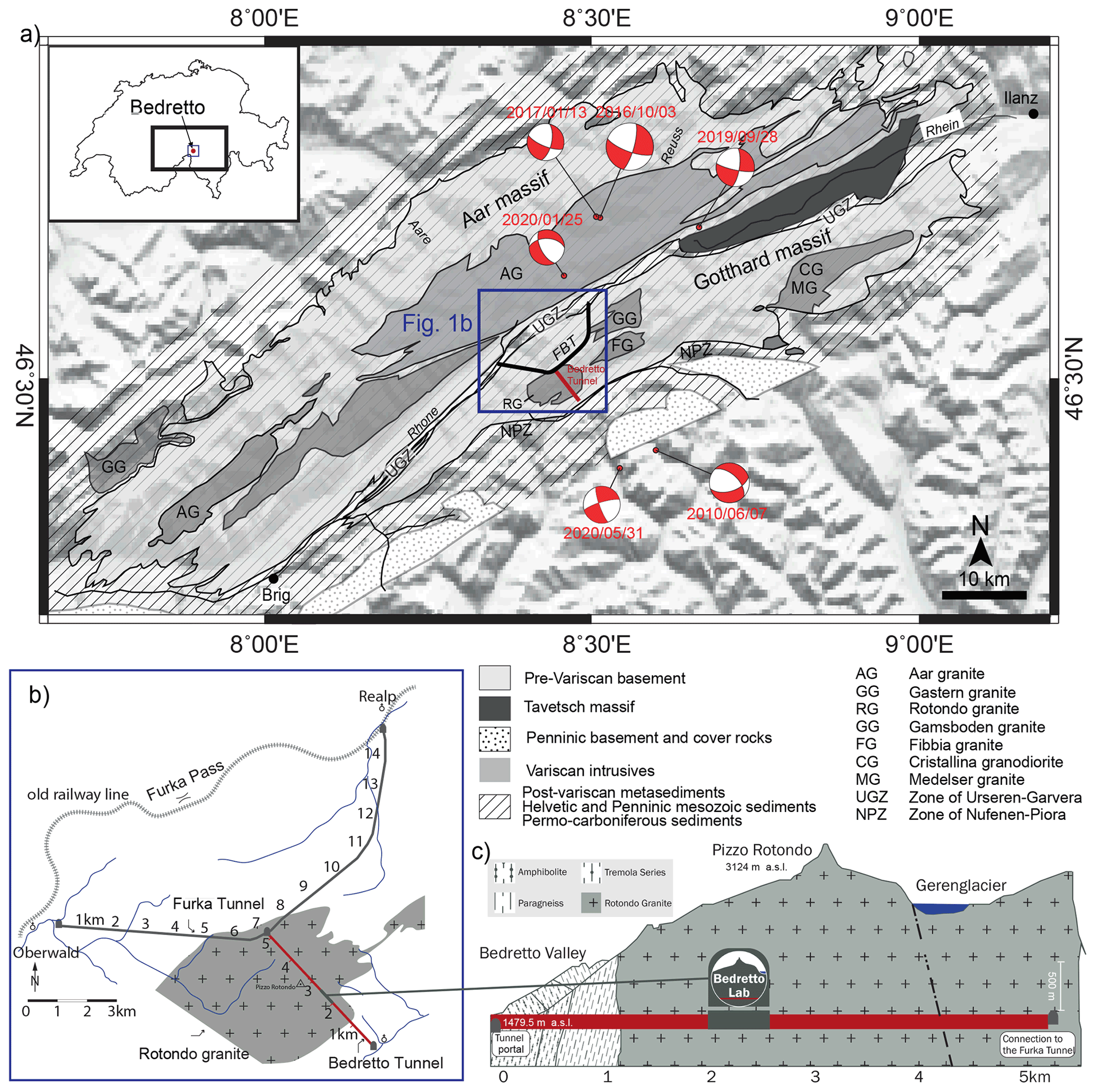 SE - Multi-disciplinary characterizations of the BedrettoLab – a new underground geoscience research facility14 fevereiro 2025
SE - Multi-disciplinary characterizations of the BedrettoLab – a new underground geoscience research facility14 fevereiro 2025 -
 Muscle dysmorphia and associated psychological features of males in the Middle East: A systematic review - ScienceDirect14 fevereiro 2025
Muscle dysmorphia and associated psychological features of males in the Middle East: A systematic review - ScienceDirect14 fevereiro 2025 -
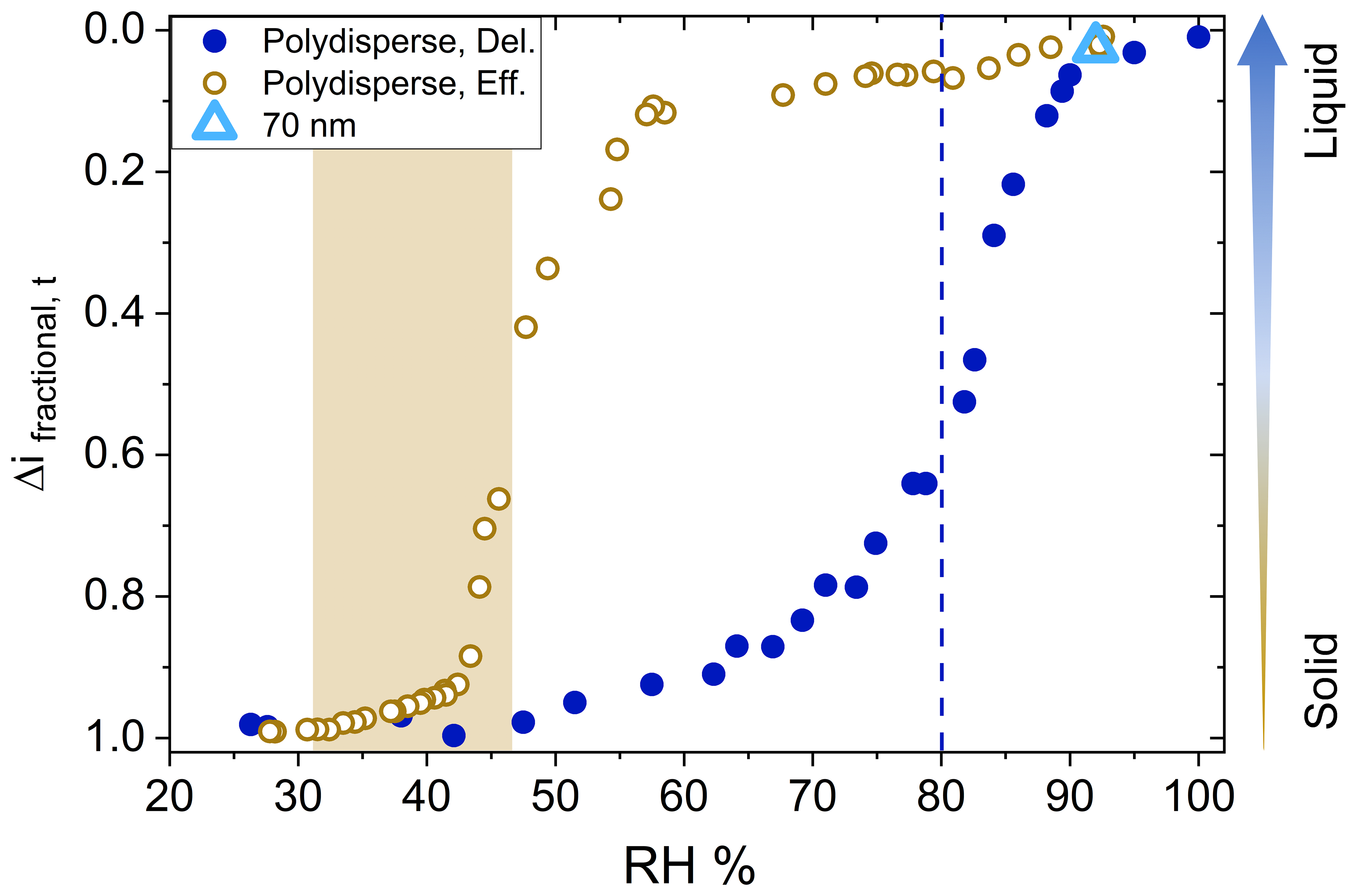 AMT - Utilizing an electrical low-pressure impactor to indirectly probe water uptake via particle bounce measurements14 fevereiro 2025
AMT - Utilizing an electrical low-pressure impactor to indirectly probe water uptake via particle bounce measurements14 fevereiro 2025 -
the blogspot website name mdpope2020has uploads about dangerous videos - Blogger Community14 fevereiro 2025
você pode gostar
-
 images.bauerhosting.com/legacy/media/61ae/4d42/a5e14 fevereiro 2025
images.bauerhosting.com/legacy/media/61ae/4d42/a5e14 fevereiro 2025 -
 Blood Lust (A Killing Stalking Fanfiction)14 fevereiro 2025
Blood Lust (A Killing Stalking Fanfiction)14 fevereiro 2025 -
 Ações da Americanas (AMER3) devem desabar nesta quinta; analistas veem notícia “desastrosa” para varejista14 fevereiro 2025
Ações da Americanas (AMER3) devem desabar nesta quinta; analistas veem notícia “desastrosa” para varejista14 fevereiro 2025 -
 Historical chess players hi-res stock photography and images - Alamy14 fevereiro 2025
Historical chess players hi-res stock photography and images - Alamy14 fevereiro 2025 -
 Evil Dead: The Game Reveals Deluxe & Collector's Edition As Preorders Go Live - PlayStation Universe14 fevereiro 2025
Evil Dead: The Game Reveals Deluxe & Collector's Edition As Preorders Go Live - PlayStation Universe14 fevereiro 2025 -
 Nintendo Switch usado? Riscos e cuidados na hora de comprar barato14 fevereiro 2025
Nintendo Switch usado? Riscos e cuidados na hora de comprar barato14 fevereiro 2025 -
 CODENAMES: A SUPREMACIA GABS! A SUPERAÇÃO DO MAIOR DE TODOS c/ Guinas, Vx, Coelho e +14 fevereiro 2025
CODENAMES: A SUPREMACIA GABS! A SUPERAÇÃO DO MAIOR DE TODOS c/ Guinas, Vx, Coelho e +14 fevereiro 2025 -
 State of Decay 3 Revealed at Xbox Games Showcase14 fevereiro 2025
State of Decay 3 Revealed at Xbox Games Showcase14 fevereiro 2025 -
 sonic.exe meets russian alphabet lore - Comic Studio14 fevereiro 2025
sonic.exe meets russian alphabet lore - Comic Studio14 fevereiro 2025 -
 Haris on X: Reminder that Neil Druckmann is a Zionist / X14 fevereiro 2025
Haris on X: Reminder that Neil Druckmann is a Zionist / X14 fevereiro 2025