Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 17 março 2025

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.

Drug development - Wikipedia

Why 90% of clinical drug development fails and how to improve it
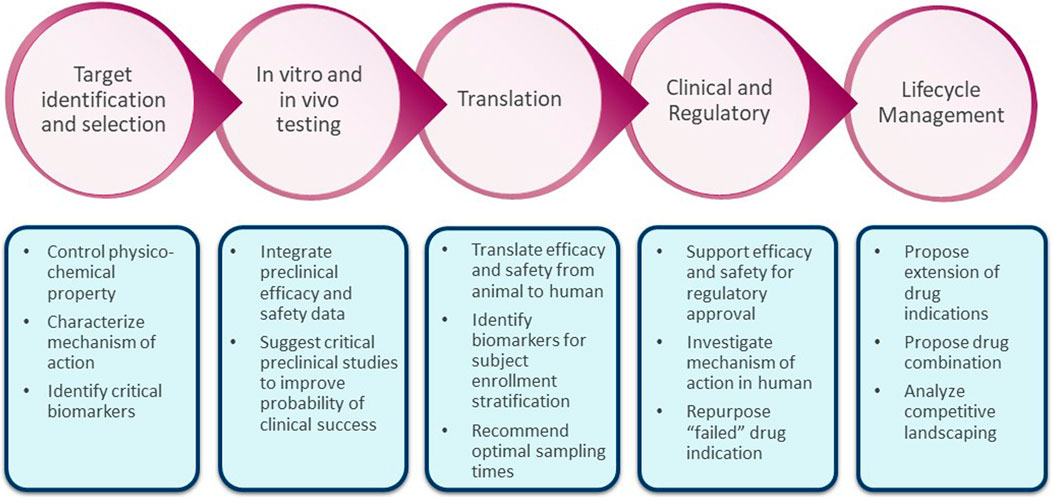
Frontiers Quantitative systems modeling approaches towards model
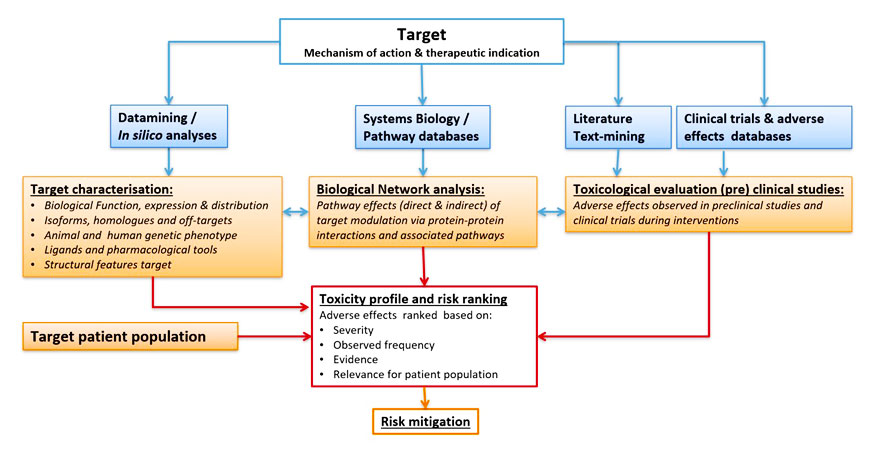
Target Profiling

Developing Role for Artificial Intelligence in Drug Discovery in
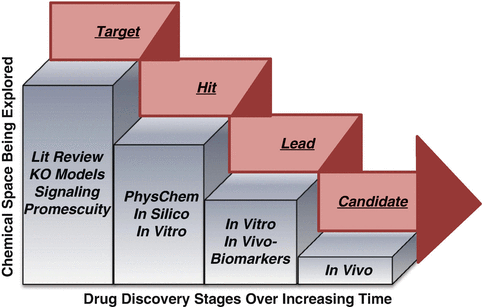
Discover Toxicology: An Early Safety Assessment Approach

PDF) Molecular clinical safety intelligence: a system for bridging
Certara, Blog

Early Phase Drug Development Solutions
Early Drug Discovery and Development Guidelines: For Academic

Eurofins Advinus BioPharma Services - Eurofins Scientific

Health economic considerations for early drug discovery - Drug
Recomendado para você
-
 Brain Test Level 411-415 Walkthrough17 março 2025
Brain Test Level 411-415 Walkthrough17 março 2025 -
 brain test Archives - Page 8 of 2817 março 2025
brain test Archives - Page 8 of 2817 março 2025 -
brain test level 411|Búsqueda de TikTok17 março 2025
-
The University of Alabama's Brain-Drone Race Flies Us to a Mind17 março 2025
-
 Expecting 411: Clear Answers & Smart Advice for Your Pregnancy17 março 2025
Expecting 411: Clear Answers & Smart Advice for Your Pregnancy17 março 2025 -
CONSORT diagram. CCT conventional coagulation test, VHA17 março 2025
-
 411 Adrenal Insufficiency with Dr. Atil Kargi - The Curbsiders17 março 2025
411 Adrenal Insufficiency with Dr. Atil Kargi - The Curbsiders17 março 2025 -
 Studies add details about the brain, clues for future treatments17 março 2025
Studies add details about the brain, clues for future treatments17 março 2025 -
 Pin on Health17 março 2025
Pin on Health17 março 2025 -
 Brain Mapping and Functional Brain Imaging Ling 411 – ppt download17 março 2025
Brain Mapping and Functional Brain Imaging Ling 411 – ppt download17 março 2025
você pode gostar
-
 How to EXIT the Shovel Cave Sons of the Forest17 março 2025
How to EXIT the Shovel Cave Sons of the Forest17 março 2025 -
 Onde E Como Jogar Paciência Spider? - Conheça Os Melhores17 março 2025
Onde E Como Jogar Paciência Spider? - Conheça Os Melhores17 março 2025 -
 7 palavras japonesas que podem nos ajudar a ter serenidade - BBC News Brasil17 março 2025
7 palavras japonesas que podem nos ajudar a ter serenidade - BBC News Brasil17 março 2025 -
 Overlord III Dublado - Episódio 11 - Animes Online17 março 2025
Overlord III Dublado - Episódio 11 - Animes Online17 março 2025 -
DOORS Monsters PACK - Roblox17 março 2025
-
/i.s3.glbimg.com/v1/AUTH_da025474c0c44edd99332dddb09cabe8/internal_photos/bs/2022/5/c/Fz1GaLTNGPIA2FFwDxKA/alemao.png) Influencer detido em operação contra rifas ilegais ostenta carros de luxo nas redes17 março 2025
Influencer detido em operação contra rifas ilegais ostenta carros de luxo nas redes17 março 2025 -
Tech Deck - Pack 2 mini skates de dedo versão Versus - Element, Concentra17 março 2025
-
 Surgeon Simulator 2: Surgery Gameplay Trailer17 março 2025
Surgeon Simulator 2: Surgery Gameplay Trailer17 março 2025 -
 30 livros conjunto livro de cópia erin hunter warriors temporada 1-5 coleção conjunto criança juventude literatura animal romance livro de ficção inglês - AliExpress17 março 2025
30 livros conjunto livro de cópia erin hunter warriors temporada 1-5 coleção conjunto criança juventude literatura animal romance livro de ficção inglês - AliExpress17 março 2025 -
 Miss Fortune ARAM Build - Wild Rift Guides17 março 2025
Miss Fortune ARAM Build - Wild Rift Guides17 março 2025