Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 12 fevereiro 2025

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Hepatitis B Foundation
Assembly Bio breaks off hepatitis B deal with Antios Therapeutics

Biotech Fierce Biotech

Antios hep B drug slows viral rebound, but FDA hold casts shadow

Real world single center experience on the efficacy of stopping

Former J&J R&D chief Mathai Mammen lands at FogPharma

Annalee Armstrong - Journalist Profile - Intelligent Relations

Materials about hepatitis B

Hepatitis B Foundation

Landon Loving on LinkedIn: Selecta, Sobi rout gout in pair of
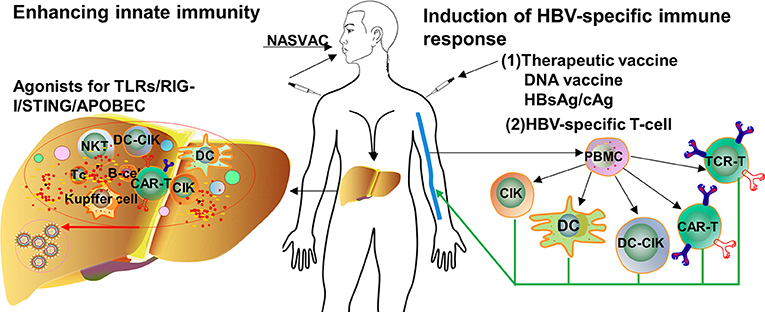
Frontiers Advances in Targeting the Innate and Adaptive Immune

IHEP (International Hepatology Education Program)

IHEP (International Hepatology Education Program)
Recomendado para você
-
 Doors (Video Game) - TV Tropes12 fevereiro 2025
Doors (Video Game) - TV Tropes12 fevereiro 2025 -
 Roblox Doors Hotel+ Addon for Minecraft12 fevereiro 2025
Roblox Doors Hotel+ Addon for Minecraft12 fevereiro 2025 -
 TWO CRUCIFIX IN ONE ROOM?! (Doors Hotel+)#shorts #roblox #doors in 202312 fevereiro 2025
TWO CRUCIFIX IN ONE ROOM?! (Doors Hotel+)#shorts #roblox #doors in 202312 fevereiro 2025 -
STRANGER THINGS HAVE HAPPENED_JIM HOPPER - 059 - Wattpad12 fevereiro 2025
-
 Here Come the Zoomers: Silicon Valley Greets a New Generation of Teen Founders — The Information12 fevereiro 2025
Here Come the Zoomers: Silicon Valley Greets a New Generation of Teen Founders — The Information12 fevereiro 2025 -
 The Free-College Fantasy12 fevereiro 2025
The Free-College Fantasy12 fevereiro 2025 -
 Online Shopping Post Covid Misread by (AMZN), Others - Bloomberg12 fevereiro 2025
Online Shopping Post Covid Misread by (AMZN), Others - Bloomberg12 fevereiro 2025 -
Halt and Catch Fire TO THE MAX12 fevereiro 2025
-
 kftI9GR.gif12 fevereiro 2025
kftI9GR.gif12 fevereiro 2025 -
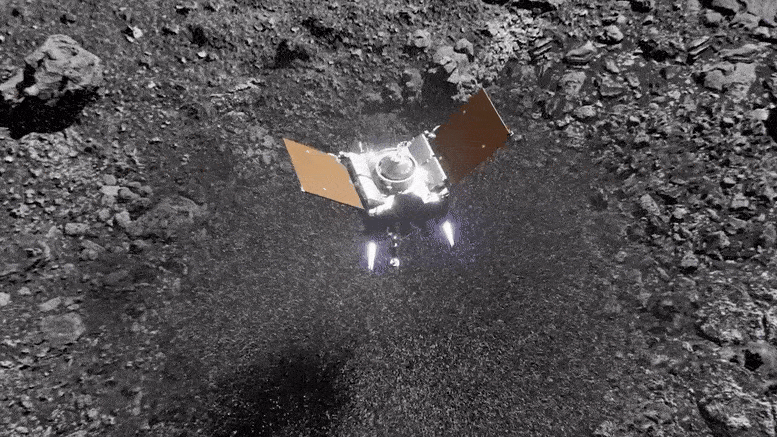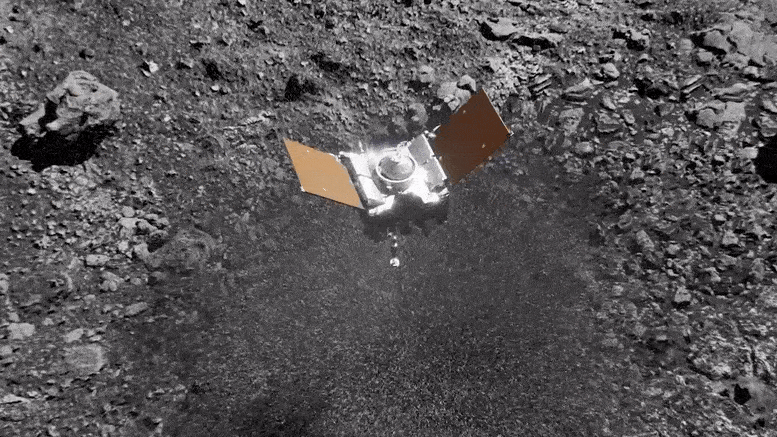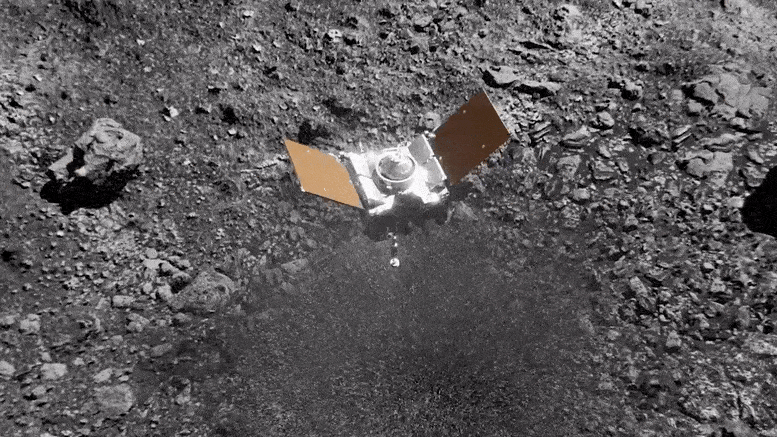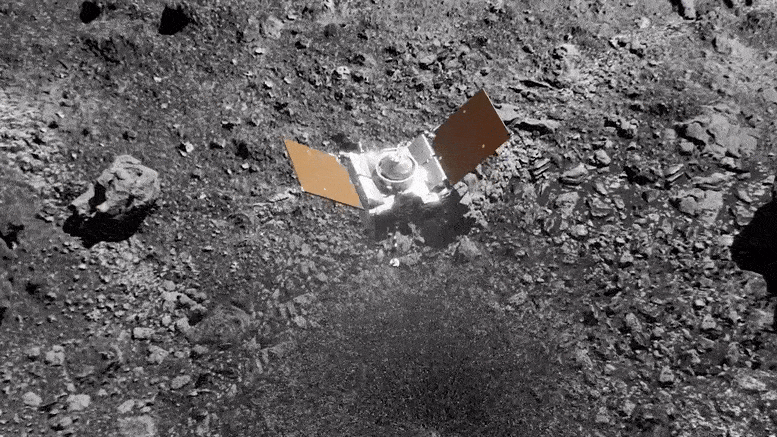 Preparing for Asteroid Bennu: NASA's OSIRIS-REx Astromaterials Lab Opens Doors to Media12 fevereiro 2025
Preparing for Asteroid Bennu: NASA's OSIRIS-REx Astromaterials Lab Opens Doors to Media12 fevereiro 2025
você pode gostar
-
 Jogos INCRÍVEIS para quem gosta de RPG de Mesa!12 fevereiro 2025
Jogos INCRÍVEIS para quem gosta de RPG de Mesa!12 fevereiro 2025 -
 Pop Queen's Gambit Beth Finale Vinyl Figure : Funko12 fevereiro 2025
Pop Queen's Gambit Beth Finale Vinyl Figure : Funko12 fevereiro 2025 -
 CapCut_bug noir francês12 fevereiro 2025
CapCut_bug noir francês12 fevereiro 2025 -
 ✨MEW + MEWTWO Shiny 6IV✨ Pokemon Scarlet & Violet Legendary 212 fevereiro 2025
✨MEW + MEWTWO Shiny 6IV✨ Pokemon Scarlet & Violet Legendary 212 fevereiro 2025 -
 Video Game Arcade – ZDT's Amusement Park12 fevereiro 2025
Video Game Arcade – ZDT's Amusement Park12 fevereiro 2025 -
 Kochi-based arm wrestler beats 'world's strongest bodybuilder12 fevereiro 2025
Kochi-based arm wrestler beats 'world's strongest bodybuilder12 fevereiro 2025 -
 Nate Not Knowing Kuybi's Name - Yo Kai Watch12 fevereiro 2025
Nate Not Knowing Kuybi's Name - Yo Kai Watch12 fevereiro 2025 -
format(webp)) Dr. STONE New World English Dub Reveals Cast & Crew, Release Date - Crunchyroll News12 fevereiro 2025
Dr. STONE New World English Dub Reveals Cast & Crew, Release Date - Crunchyroll News12 fevereiro 2025 -
 Lightning McQueen Adult Crocs Sell Out, Chaos Ensues12 fevereiro 2025
Lightning McQueen Adult Crocs Sell Out, Chaos Ensues12 fevereiro 2025 -
 CapCut_how to play fnaf 1 for free12 fevereiro 2025
CapCut_how to play fnaf 1 for free12 fevereiro 2025

