FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 16 março 2025

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
FDA Meeting on COVID-19 Vaccine and Emergency Use Authorization
FDA Open Meeting on Johnson & Johnson COVID-19 Vaccine, Part 4

FDA withdraws emergency use authorization of COVID drug because it

FDA Advisory Committee Votes to Recommend Pfizer COVID-19 Vaccine

Federal Register :: Authorizations of Emergency Use of Certain
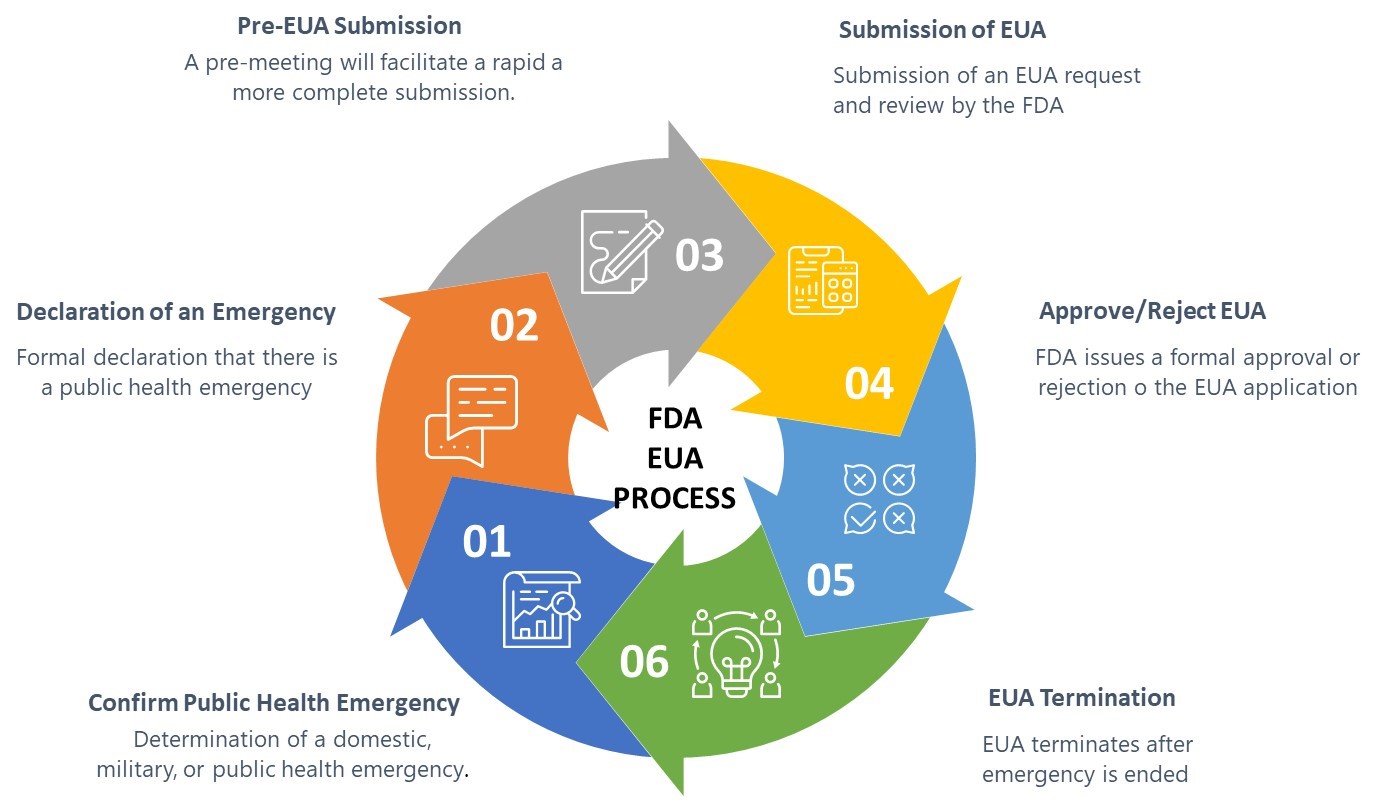
Navigating The FDA's Emergency Use Authorization Process
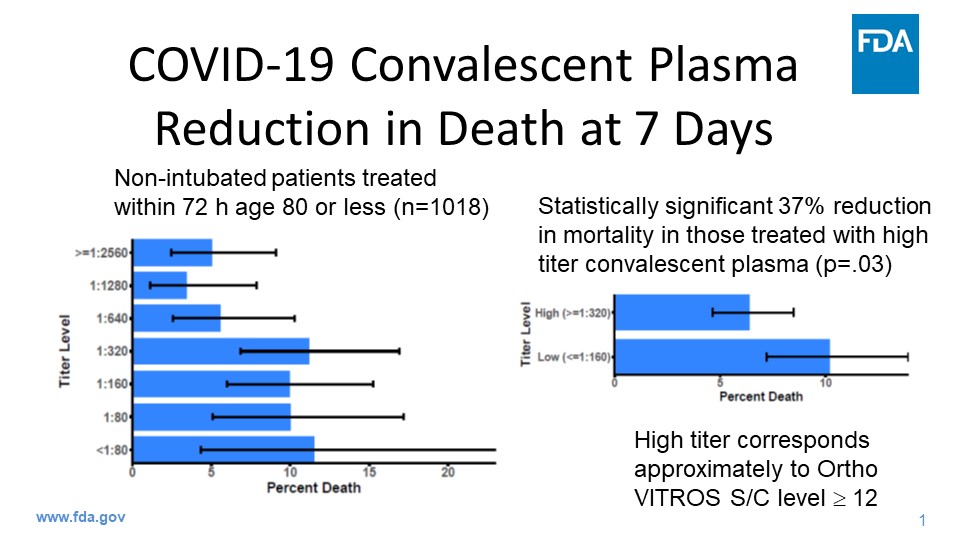
FDA Issues Emergency Use Authorization for Convalescent Plasma as

Federal Register :: Authorizations of Emergency Use of Certain
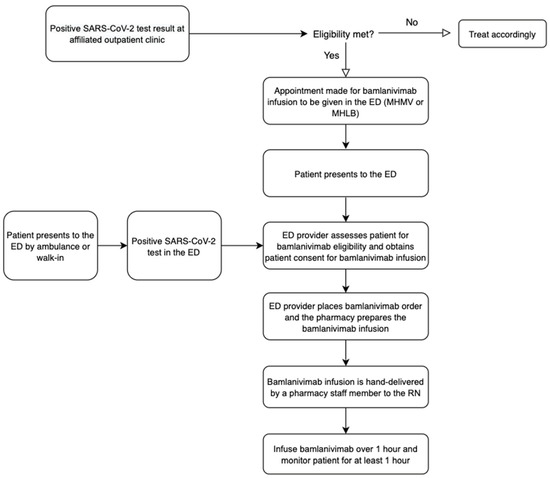
Healthcare, Free Full-Text
Vir (VIR) Stock Falls After FDA Narrows Guidance on Covid Antibody

FDA won't comment on status of Emergency Use Authorizations for

IJMS, Free Full-Text
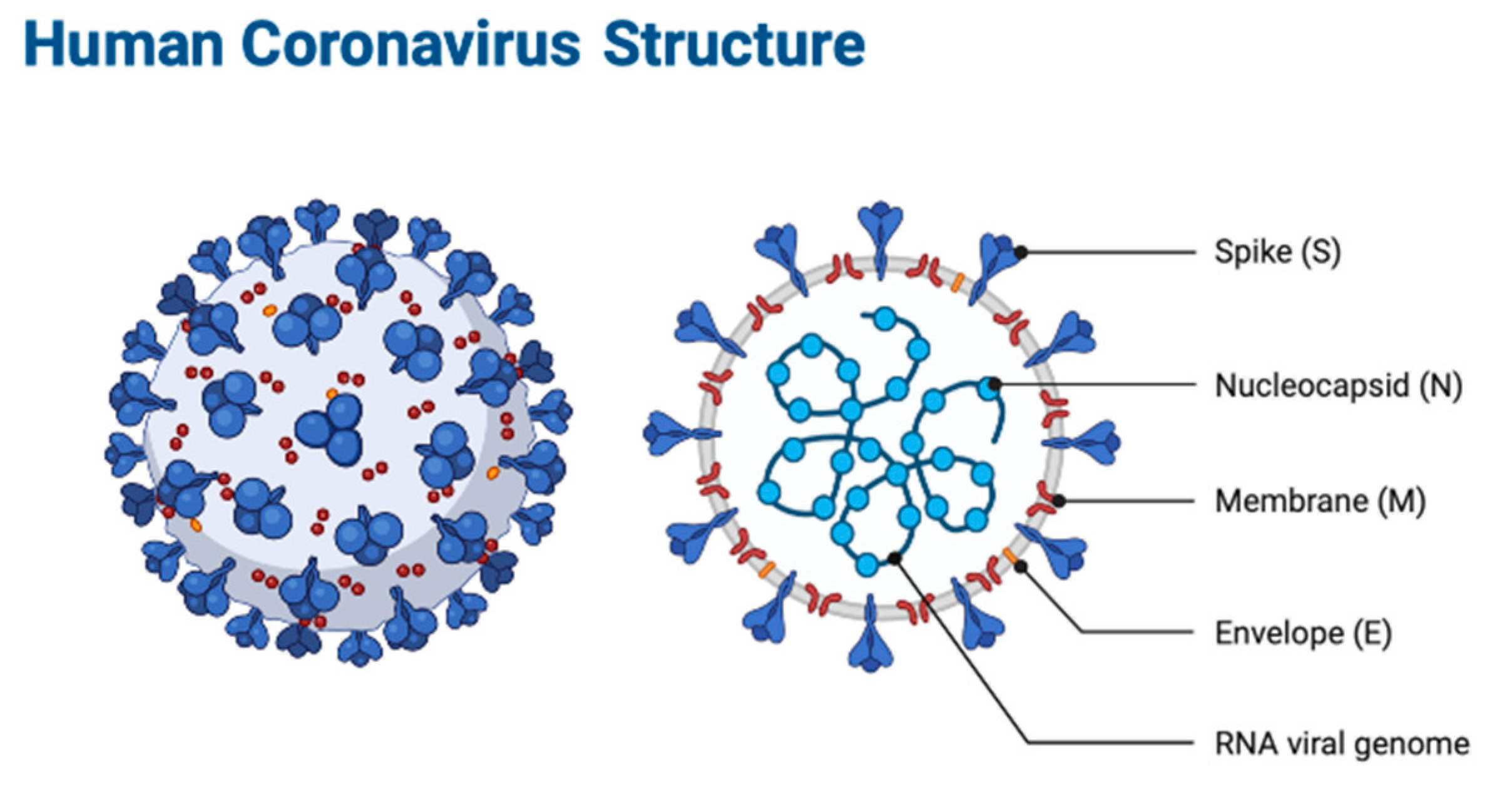
IJMS, Free Full-Text
Recomendado para você
-
 Agustín Fernández Mallo's The Things We've Seen Holds a Trick16 março 2025
Agustín Fernández Mallo's The Things We've Seen Holds a Trick16 março 2025 -
 Isaac Azar, MD, Aventura, FL Emergency Medicine Physician16 março 2025
Isaac Azar, MD, Aventura, FL Emergency Medicine Physician16 março 2025 -
The Noite (@SBTTheNoite) / X16 março 2025
-
 2019 Scientific Program - 2019 - Journal of Ultrasound in Medicine - Wiley Online Library16 março 2025
2019 Scientific Program - 2019 - Journal of Ultrasound in Medicine - Wiley Online Library16 março 2025 -
 Paris 6 restaurant hi-res stock photography and images - Page 3 - Alamy16 março 2025
Paris 6 restaurant hi-res stock photography and images - Page 3 - Alamy16 março 2025 -
 ABFAS 2022 Newsletter by ABFAS - Issuu16 março 2025
ABFAS 2022 Newsletter by ABFAS - Issuu16 março 2025 -
BUFFET COMPLETASSO COM OPEN DE HÄAGEN-DAZS E NUTELLA 😱😱 Fala Desbra16 março 2025
-
 Antonia Fontenelle não liga para críticas à sexualidade de Sheik16 março 2025
Antonia Fontenelle não liga para críticas à sexualidade de Sheik16 março 2025 -
 With 'Gill's Electronic Theory of Magnetism 1964' – Book Store – B P International16 março 2025
With 'Gill's Electronic Theory of Magnetism 1964' – Book Store – B P International16 março 2025 -
Current Residents, Internal Medicine Residency, Washington, DC16 março 2025
você pode gostar
-
 Rolls-Royce Ghost Review, Interior, For Sale, Specs & Models in Australia16 março 2025
Rolls-Royce Ghost Review, Interior, For Sale, Specs & Models in Australia16 março 2025 -
 Marvel vai lançar pela primeira vez quatro filmes no mesmo ano - Monet16 março 2025
Marvel vai lançar pela primeira vez quatro filmes no mesmo ano - Monet16 março 2025 -
 Análise: Novo PlayStation Plus traz um catálogo recheado de jogos16 março 2025
Análise: Novo PlayStation Plus traz um catálogo recheado de jogos16 março 2025 -
 Bleach Original Production Sketch Genga Douga Set Muramasa16 março 2025
Bleach Original Production Sketch Genga Douga Set Muramasa16 março 2025 -
 Stream GTA - Vice City ( MIDEDIT Remake ) FREE DOWNLOAD by MIDEDIT16 março 2025
Stream GTA - Vice City ( MIDEDIT Remake ) FREE DOWNLOAD by MIDEDIT16 março 2025 -
Ids de roupas do brok16 março 2025
-
slenders would also be less hated if 2020 was more like 202316 março 2025
-
 Papel De Arroz Bento Cake Flork Meme Odeio Drama Mas Faço - Mec16 março 2025
Papel De Arroz Bento Cake Flork Meme Odeio Drama Mas Faço - Mec16 março 2025 -
 Vector Jumping Dino Stock Illustration - Download Image Now16 março 2025
Vector Jumping Dino Stock Illustration - Download Image Now16 março 2025 -
 Novo trailer dublado de Naruto x Boruto: Ultimate Ninja Storm Connections destaca os personagens jogáveis16 março 2025
Novo trailer dublado de Naruto x Boruto: Ultimate Ninja Storm Connections destaca os personagens jogáveis16 março 2025



